2-butanone (1) +
water (2) binary
(i)
VLE
type: two-liquid phase forming mixture
(ii)
Vapor
pressures:
p1s=
42.1*exp(para1*tclap**3+para2*tclap**2+para3*tclap) [bar]
para1=0.166255372094337
para2=-0.338357600295482
para3=6.91698751947342
tclap=1-1/(T/536.8)
T: temperature [K]
p2s=221.2*exp(para1*tclap**3+para2*tclap**2+para3*tclap)
[bar]
para1=0.222438359031832
para2=-0.0522401918107863
para3=7.17725676105391
tclap=1-1/(T/647.3)
T: temperature [K]
(iii)
y1,2L =aT**2+bT+c (0 deg C < T < 125 deg C)
a=-2.72748693
b=-0.002686283
c=0.8306088
T: temperature
[deg C]
The
values, T and y1,2L, denote temperature and y1 value of the
two-liquid phase forming mixture and y1 is the composition of component 1 in
the vapor phase.
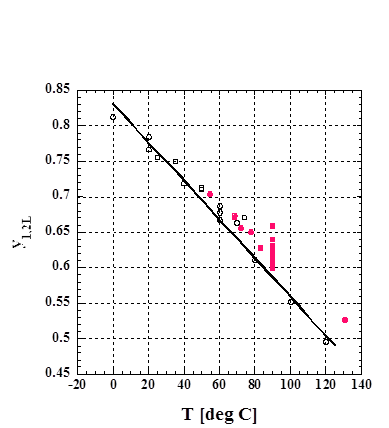
Fig. 2
y1,2L vs T for the 2-butanone (1)
+ water (2) binary, (○) y1,2L determined from constant
temperature VLE data, (●) y1,2L determined from constant
pressure data, and (−) predicted using the PPM.