Methanol (1) +
benzene (2) binary
(i)
VLE
type: minimum azeotropic mixture
(ii)
Vapor
pressures:
p1s=80.9*exp(para1*tclap**3+para2*tclap**2+para3*tclap)
[bar]
para1=0.212768828771579
para2=-0.319352375891615
para3=8.23174708919747
tclap=1-1-/(T/512.6)
T: temperature [K]
p2s=
48.9*exp(para1*tclap**3+para2*tclap**2+para3*tclap) [bar]
para1=0.489662525497929
para2=0.181629447193235
para3=6.49827277766285
tclap=1-1/(T/562.3)
T: temperature [K]
(iii)
y1,azeo =aT**2+bT+c (0 deg C < T < 121 deg C)
a=-5.2209835
b=0.002462834
c=0.48346497
T: temperature [deg C]
The
values, T and y1,azeo, denote temperature and y1 value of azeotropic
mixture where y1 is the composition of component 1 in the vapor phase.
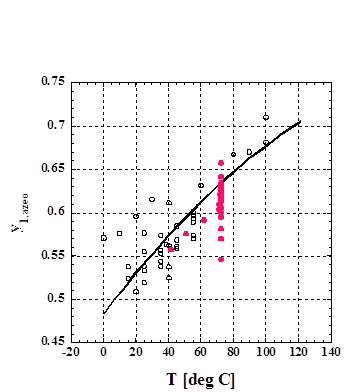
Fig. 1
y1,azeo vs. T for the methanol
(1) + benzene (2) binary, (○) y1,azeo determined from
constant temperature VLE data, (●) y1,azeo
determined from constant pressure data, and (−) predicted using
the PPM.